USP 797
While a good portion of the USP 797 guidelines pertain to improving air quality in these facilities, an equally important goal is to prevent physical contact with and contamination of these preparations during manufacture. Products are manufactured according to risk levels: low, medium or high. Products that are to be injected carry the greatest risk of serious health effects; therefore these products must be manufactured in an area having the lowest risk level for contamination. The lowest risk level required under USP 797 for a critical area is an ISO Class 5 area designation.
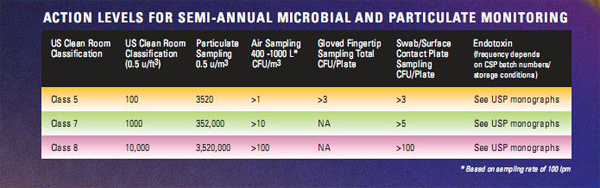
The design of Class 5 clean areas for preparation and Class 7 buffer areas, surrounding Class 5 areas, is a requirement. Semi-annual monitoring for viable bacteria and fungi in air, gloved fingertip, surface contact plates and particulates is required for both Class 5 and Class 7 designated areas.
These monitoring results trended over time will provide information on any deterioration in air quality and aseptic technique. Generally, this monitoring should be conducted semi-annually.
The guidelines impact not only the people who prepare the compounded sterile pharmaceuticals (CSPs) but also the areas where theses drugs are prepared and stored including commercial and hospital pharmacies, clinics, doctor's offices and other facilities. USP 797 recommends certain clean area designs, storage specifications, Quality Assurance plans which include environmental monitoring and employee training programs to accomplish the safe handling of these preparations.
These guidelines specifically address:
• Microbial contamination
• Endotoxin
• Physical or chemical contamination
• Preparation of incorrect concentrations
• Incorrect ingredients
A mistake in any of these areas may cause serious injury or even death.